Helium Helium Valence Electrons
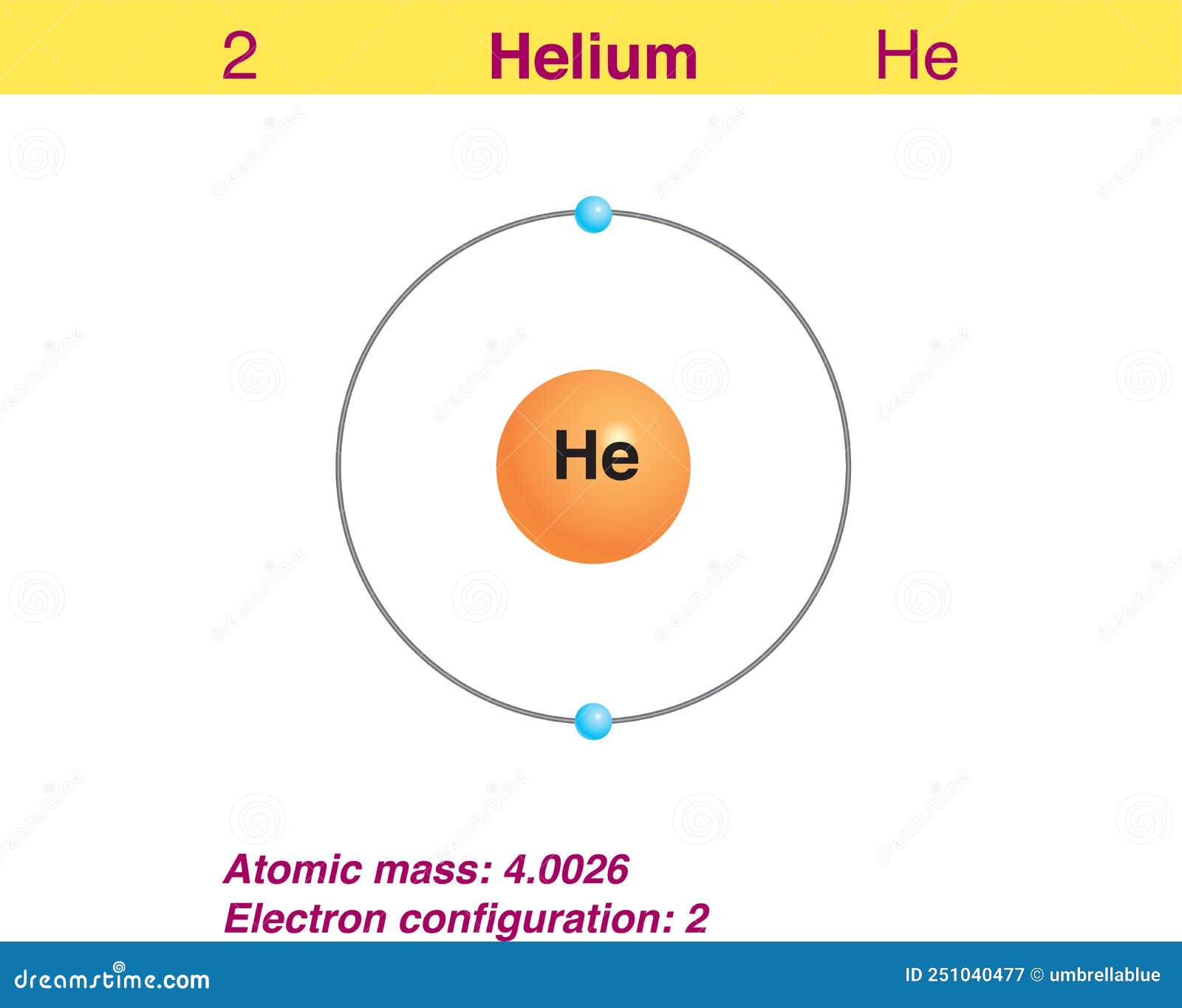
Electron of the Element Helium Stock Vector Illustration of electron
There are two ways to find the number of valence electrons in Helium (He). The first is to use the Periodic Table to figure out how many electrons Helium has.
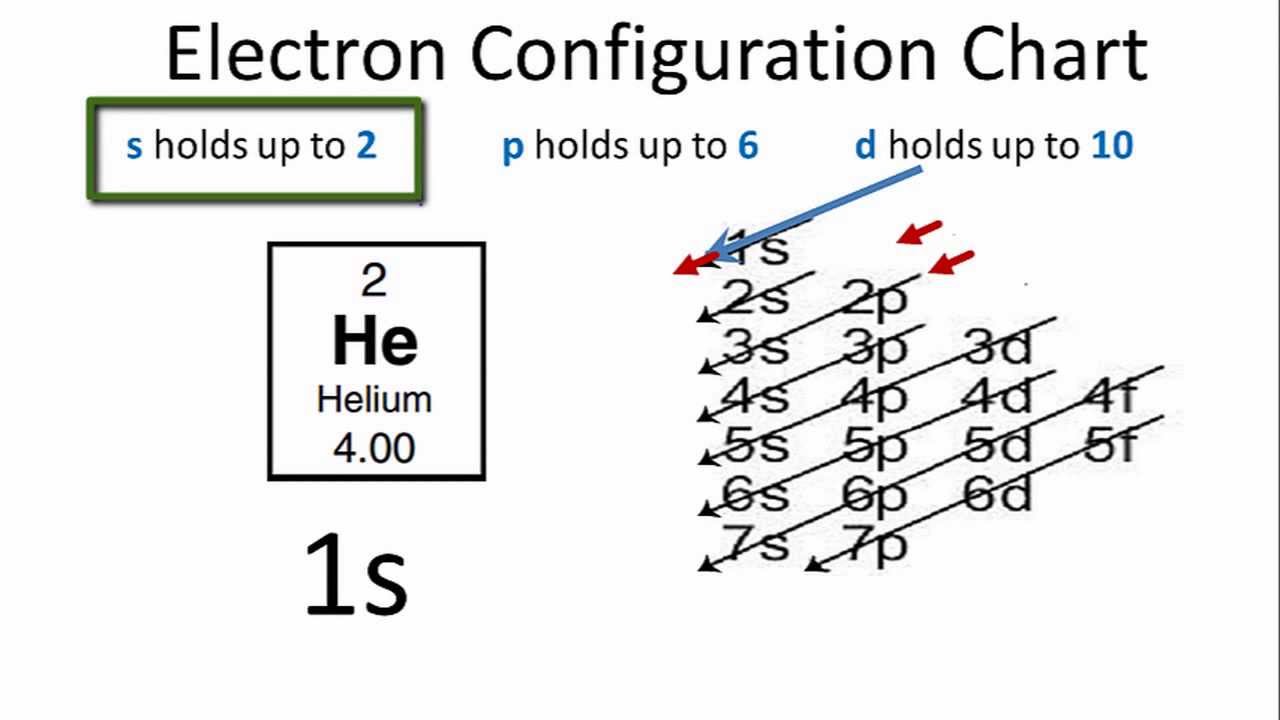
How To Find the Helium Electron Configuration (He)
Helium Valence Electrons: Helium is a chemical element of the periodic table. It is the 2nd lightest element out of all other elements. It is a type of gas that converts in a liquid stage at 268.9°C. Also, it is odorless, tasteless, and colorless. It's boiling and freezing points are lower than the other existing substance.

Protons, Neutrons, Electrons for Helium Complete Guide
How many valence electrons does helium have? Chemistry The Periodic Table Valence Electrons 2 Answers Stefan V. Feb 17, 2015 2. Explanation: Helium is located in period 1, group 18 of the Periodic Table and has an atomic number equal to 2. As a result, neutral helium will only have 2 electrons surrounding its nucleus.

Helium Valence Electrons In Helium
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable.
Uses of Metals and Non Metals [in Daily life] Teachoo Concepts
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
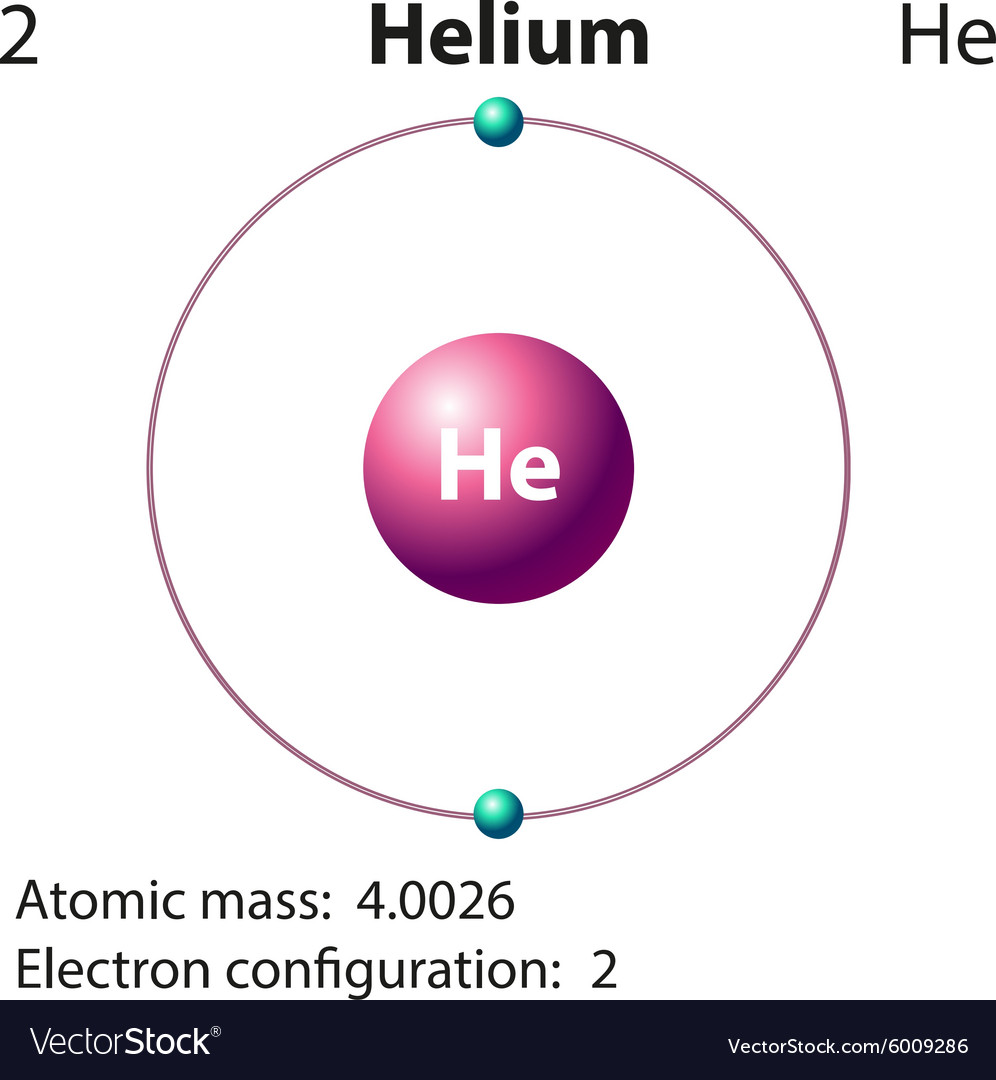
Periodic Table Helium Electron Configuration Periodic Table Timeline
Valence bond theory describes a chemical bond as the overlap of atomic orbitals. In the case of the hydrogen molecule, the 1s orbital of one hydrogen atom overlaps with the 1s orbital of the second hydrogen atom to form a molecular orbital called a sigma bond which contains two electrons of opposite spin. The mutual attraction between this.

How to find Valency? What are valence electrons? Teachoo (2023)
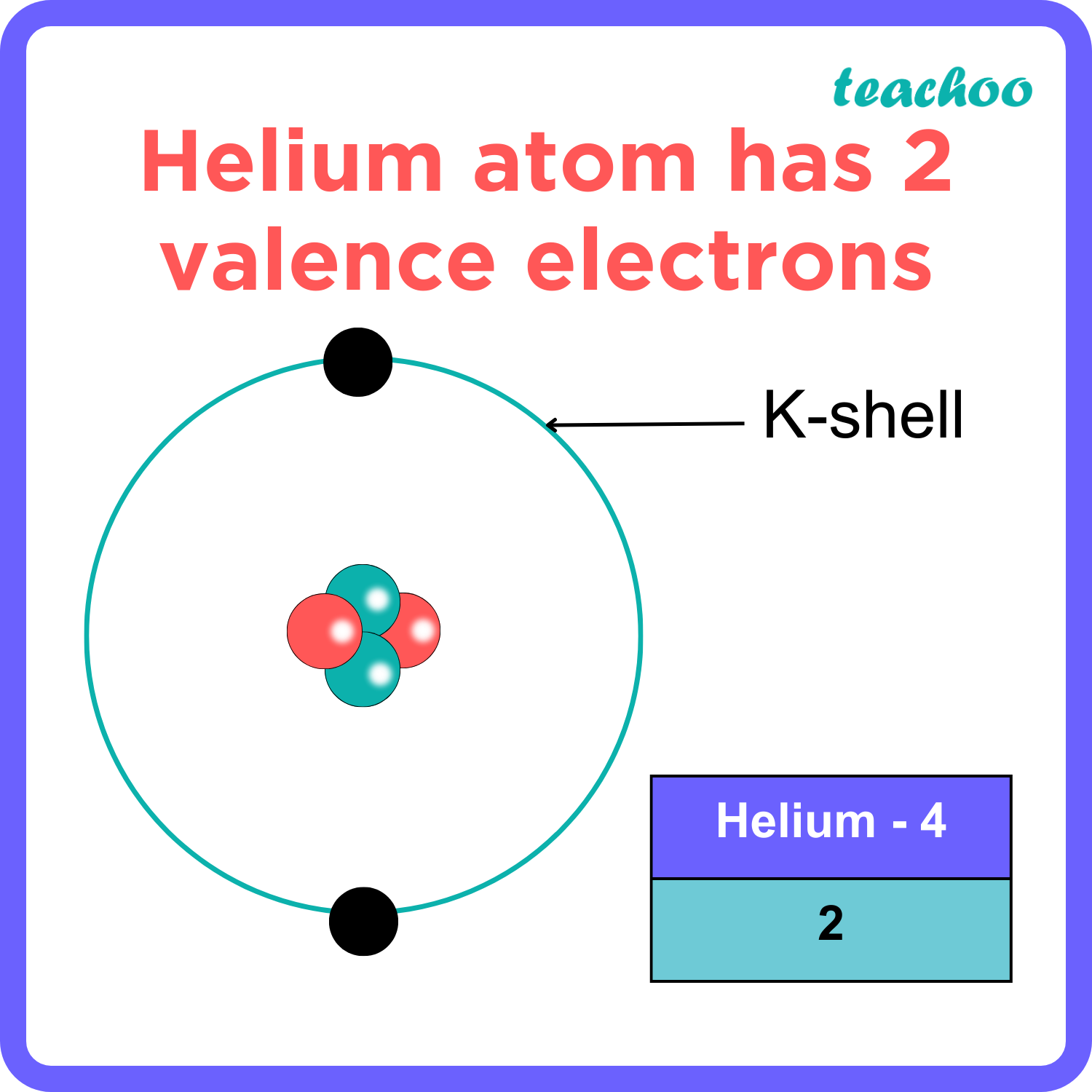
Calcium would have two valence electrons, since it is in Group IIA (Group 2). Helium is the only exception for the main group elements. The first energy level holds a maximum of two valence electrons. Since helium atoms only have two electrons and the outermost energy level is the first energy level, there can only be two valence electrons.
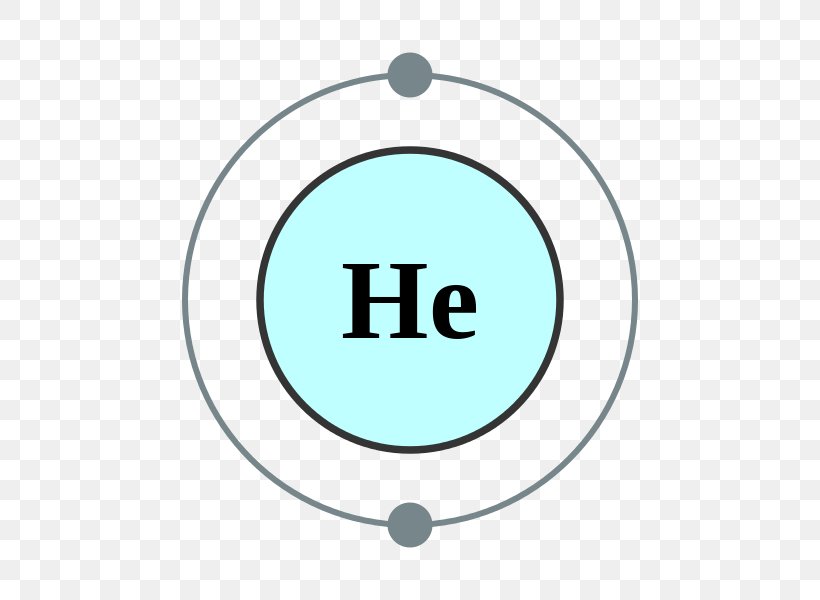
Electron Shell Helium Atom Valence Electron Electron Configuration, PNG
Explanation: Helium (He) has the highest ionization energy because, like other noble gases, helium's valence shell is full. Therefore, helium is stable and does not readily lose or gain electrons. 2. Answer: A.) True Explanation: Atomic radius increases from right to left on the periodic table. Therefore, nitrogen is larger than oxygen. 3.
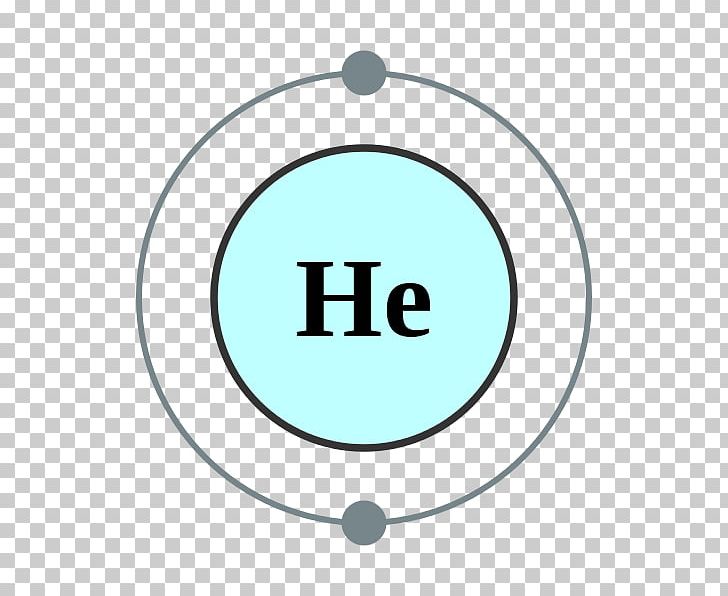
Electron Shell Helium Atom Valence Electron Electron Configuration PNG
In general, the number of valence electrons is the same within a column and increases from left to right within a row. Group 1 elements have just one valence electron and group 18 elements have eight, except for helium, which has only two electrons total. Thus, group number is a good predictor of how reactive each element will be:
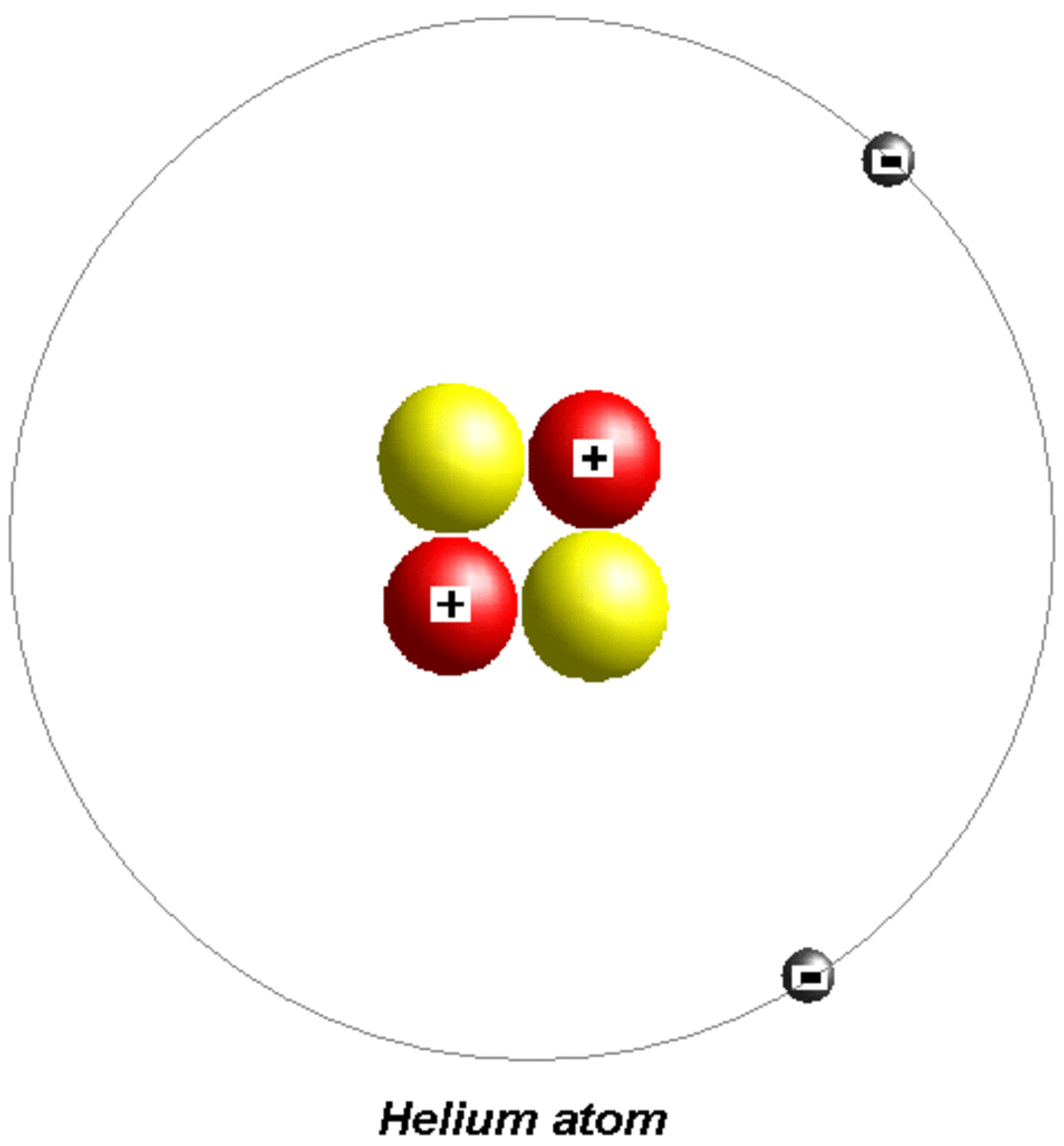
Helium Helium Valence Electrons
You can see in the electron configuration of helium ( 1s2) that the highest energy level is 1. And the total number of electrons present in this energy level is 2. So by knowing the electron configuration, we have found that the Helium has 2 valence electrons. I hope you have understood the methods of finding the valence electrons in helium.
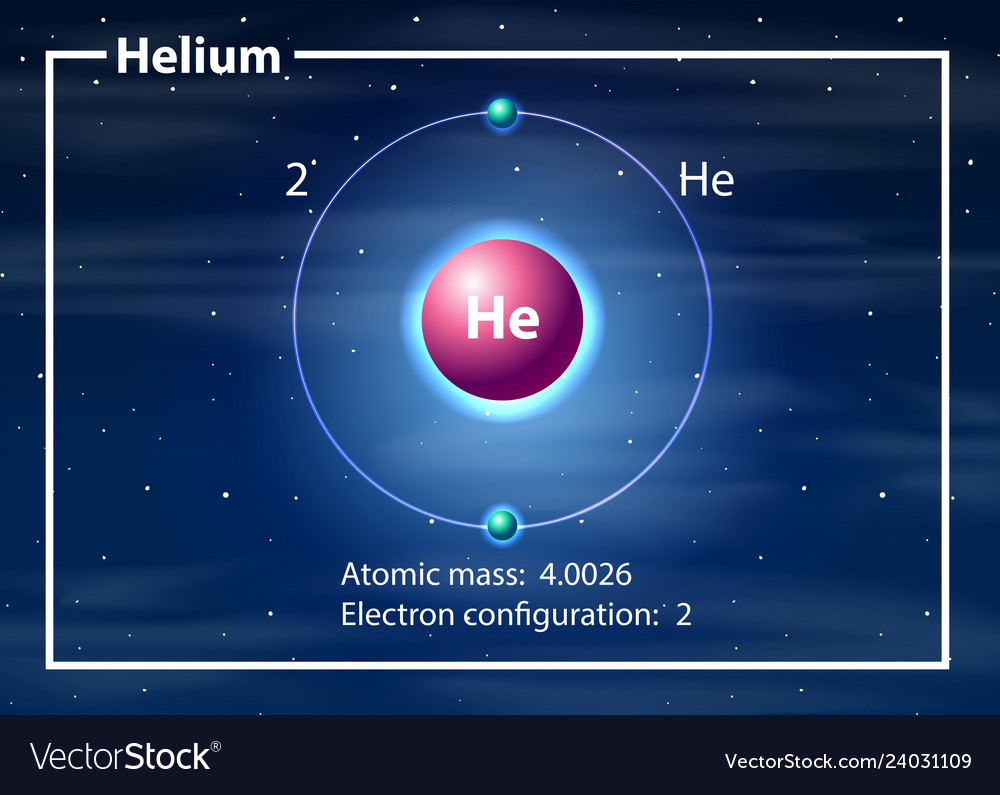
Helium atom diagram concept Royalty Free Vector Image
The electron dot diagram for helium, with two valence electrons, is as follows: \[\mathbf{He}\mathbf{:} \nonumber \] By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later.

How to Find the Valence Electrons for Helium (He) YouTube
The exception is helium, which has two valence electrons. This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons. The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons. Metals in the middle of the periodic table become more stable.
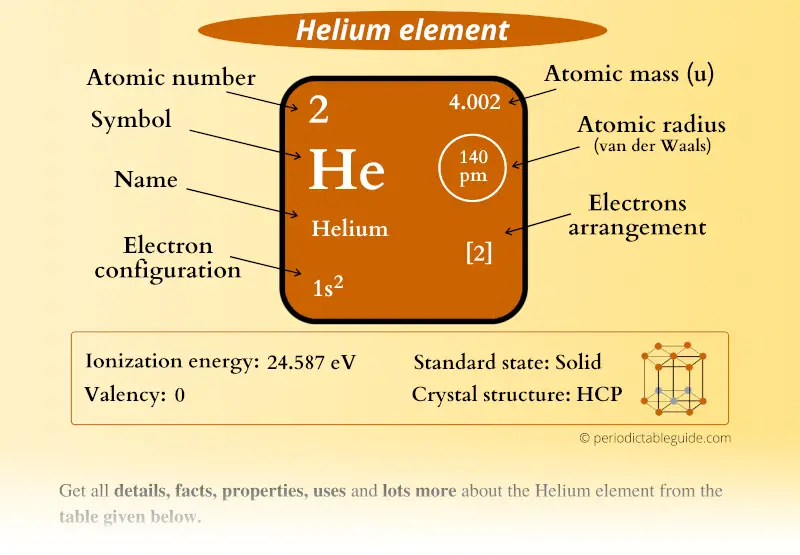
Helium Element in Periodic table (Info + Why not in group 2)
Helium is the 2nd element in the periodic table and its symbol is 'He'. In this article, I have discussed in detail how to easily write the complete electron configuration of helium. What is the electron configuration of helium? The total number of electrons in helium is two.
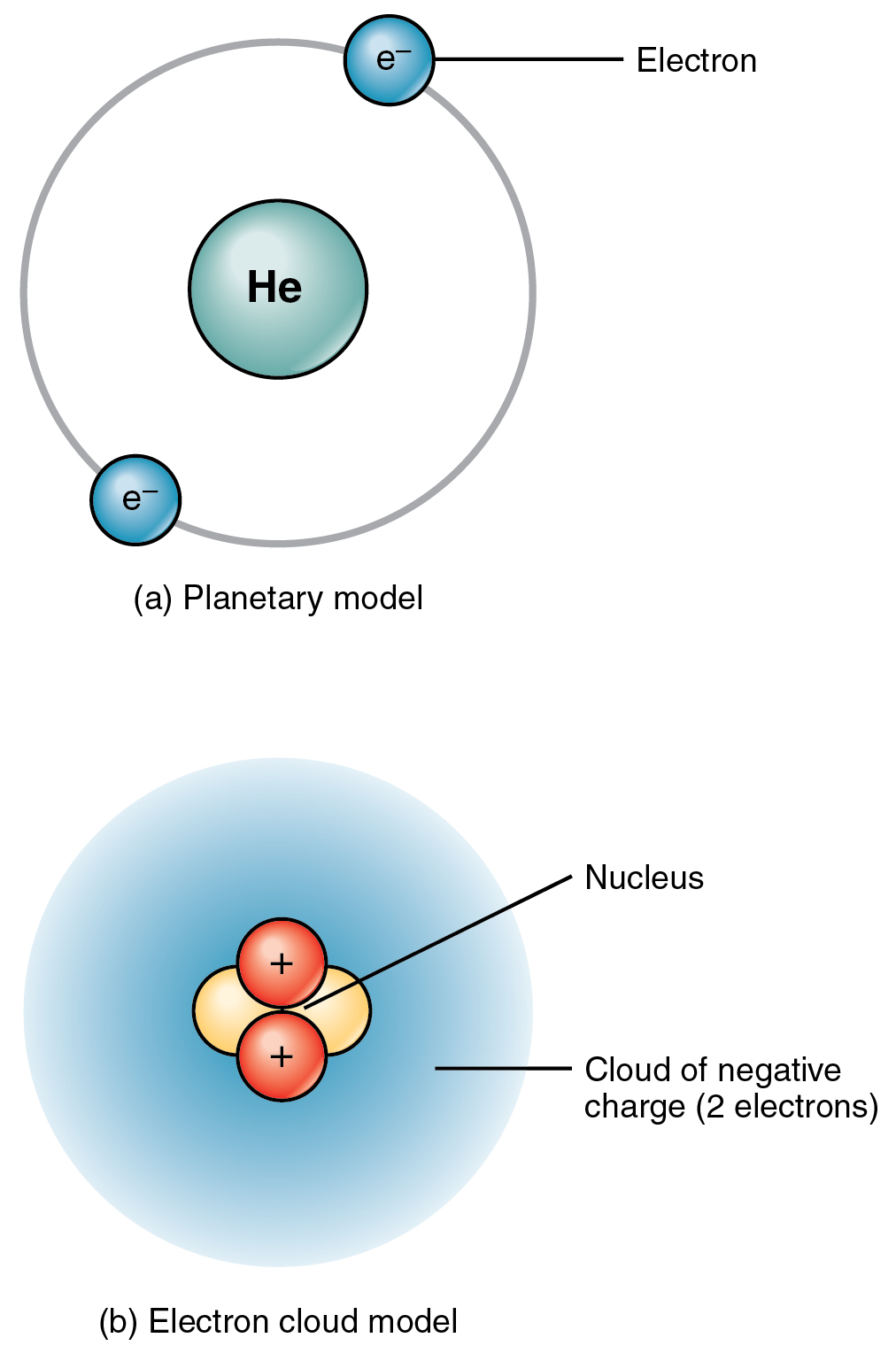
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
The number of valence electrons of helium is 2 because of its configuration which is 1s2 1 s 2. Hovewer, the valency is 0 because it is already stable and none of the outermost electrons are involved in the formation of chemical bonds. The outermost shell of an atom can accomodate a max of 8 electrons.
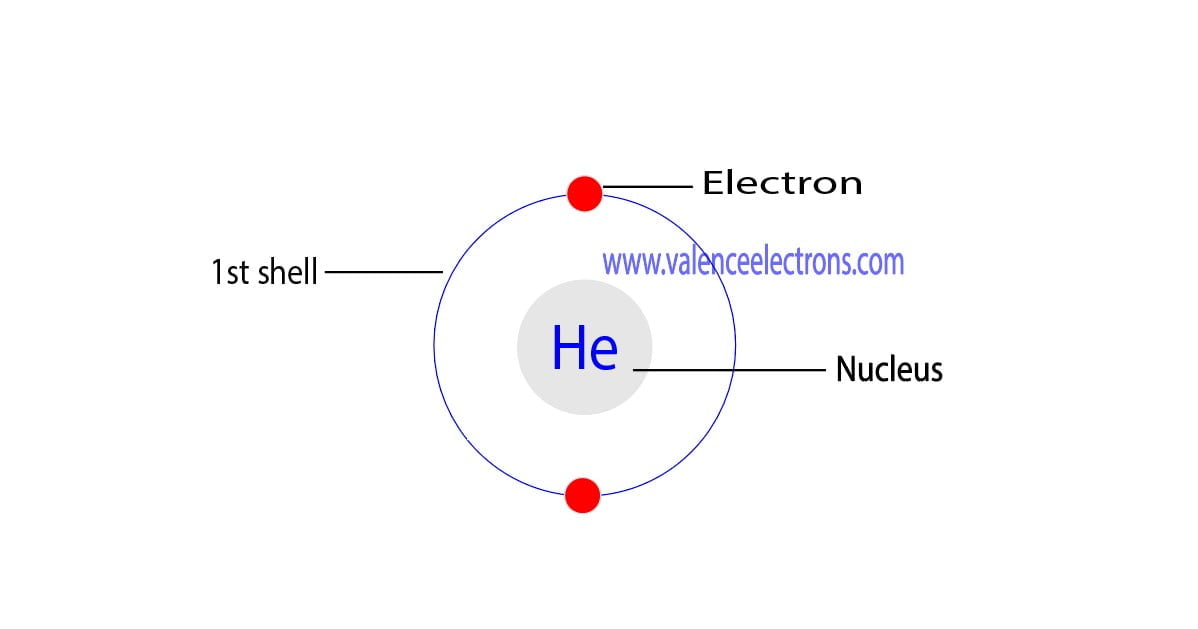
How To Find The Valence Electrons For Helium (He)?
Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons and (usually) two neutrons. As in Newtonian mechanics,. Helium II has no such valence band but nevertheless conducts heat well.
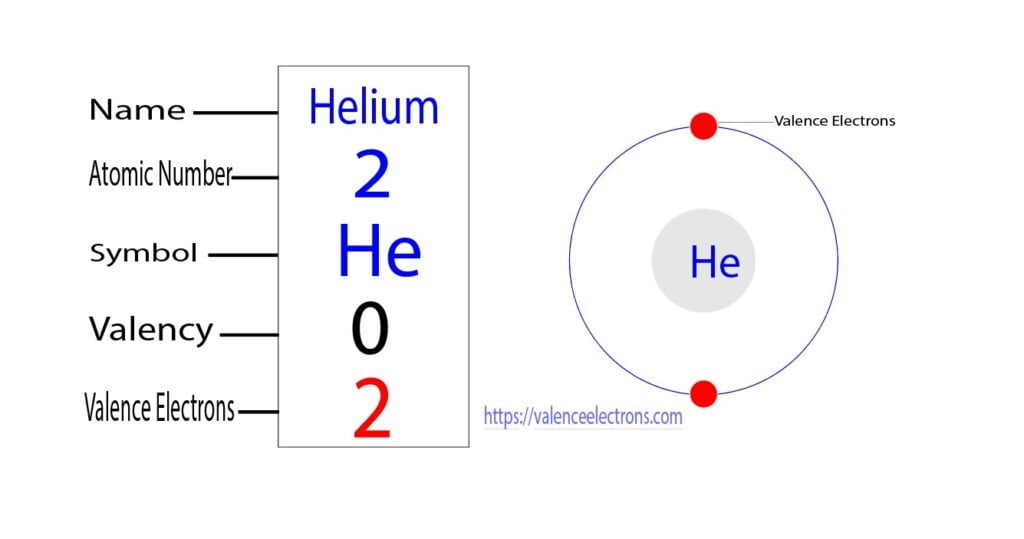
How To Find The Valence Electrons For Helium (He)?
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have.